Science and Technolgy.
科技。
Modern alchemy.
現代煉金術。
Turning a line.
進入新的一行。
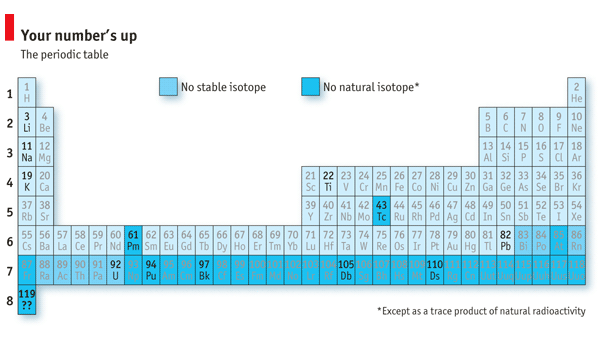
The race to extend the periodic table continues.
擴展元素周期表的比賽仍在繼續。
ONE of the first inklings that chemistry has an underlying pattern was the discovery, early in the 19th century, of lithium, sodium and potassium-known collectively as the alkali metals. Though different from each other they have strangely similar properties. This was one of the observations that led a German chemist called Johann Debereiner to wonder if all chemical elements came in families.
早在19世紀,人們對化學潛在規律已有模糊認知之時,鋰、鈉、鉀被發現,它們同被稱為堿金屬。雖然并不相同,它們卻有著不可思議的相似性質。正是這項發現,使德國化學家Johann Debereiner產生懷疑,是否所有的化學元素都是成族出現的。
It took decades to tease out the truth of Debereiner's conjecture, and thus to construct the periodic table-in which the alkali metals form the first column. And it took decades more to explain why the table works (it is to do with the way electrons organise themselves in orbit around atomic nuclei). But it is a fitting tribute to Debereiner's insight that, if all goes well, some time in the next few months will bring the creation of a new alkali metal, element number 119, by his countryman Christoph Dullmann of the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt. With that addition the table will do something which has never happened before. It will grow a new row.
科學家們證實Debereiner猜想的正確性用了數十年的時間,也因此建立了元素周期表——堿金屬組成了第一族。其后又花費了數十年的時間來解釋為何周期表是起作用的(與電子繞原子核外軌道運動的方式有關)。在即將到來的幾個月里,如果一切順利,德國達姆施塔特市GSI亥姆霍茲重離子研究中心的Christoph Dullmann將合成一個新的堿金屬,即元素周期表中第119號元素。這也恰恰彰顯了其同胞Debereiner的深刻洞察力。有了這個元素,周期表會發生前所未有的變化——延伸出新的一行。
Come in number 119.
119號元素即將出現。
An element's atomic number is the number of protons in its nucleus. These, despite being mutually repulsive because they are positively charged, are held together by a phenomenon called the strong nuclear force. Some of this force is also supplied by neutrons, which outnumber protons in most nuclei and have no electric charge. If, however, there are too many or too few neutrons in a nucleus, that nucleus becomes unstable-in other words, it is radioactive. And the vagaries of quantum physics mean that "too many" and "too few" sometimes overlap, and there is thus no stable isotope (or variant, with fewer or more neutrons) of a particular element.
一種元素的原子序數是其原子核中的質子數。雖然質子之間因帶正電荷而相互排斥,但被強核力聚集在一起。強核力的一部分也由中子提供,在大多數原子核內,中子比質子數量多,并且不帶電荷。然而,如果核內中子過多或過少,原子核將變得不穩定——即具有放射性。難以捉摸的量子力學表明,中子"太多"和"太少"有時會重疊出現,因而一些特定的元素沒有穩定的同位素(或稱變體,核內中子數不同)。
This happens at two places-islands of instability, if you like-in the middle of the table. As a result technetium, element number 43, and promethium, 61, are always radioactive (and are not found naturally in detectable quantities). Further down the table, where nuclei get heavier and elements less familiar, instability happens more and more often. No element heavier than lead (number 82) has a stable isotope, and above number 92 (uranium) lifetimes are so short that these substances are almost non-existent in nature. Such "transuranic" elements can, however, be made artificially by the fusion of lighter ones. And that is precisely what Dr Dullmann intends to do in the case of element 119, by firing titanium atoms (number 22) at those of berkelium (97) and hoping some of them merge.
這樣的不穩定元素島在周期表中部的兩處出現。锝(43號元素)和钷(61號元素)都是放射性的,并且在自然中的存在數量無法偵測。周期表中繼續向下看,元素的原子核都變得越來越重,元素也越來越不為人所熟悉,不穩定性更是頻繁出現。比鉛(82號)更重的元素都沒有穩定的同位素,在鈾(92號)之后的元素壽命太短,以致于在自然界中幾乎不存在。這些"超鈾"元素可以通過更輕元素的熔合來人工合成,這也是Dullmann博士想對119號元素應用的,將鈦(22號)原子與锫(97號)原子大量熔合,希望其中一些可以合并產生新元素。
Making a new element is tricky. The titanium atoms must be travelling fast enough in GSI's particle accelerator to overcome the repulsion between their protons and those of the berkelium, yet slowly enough to avoid ripping the newly formed atom of element 119 apart before it has had time to settle down. With the right mix, though, Dr Dullmann is confident that one or two atoms of 119 will be created over the course of the next few months, and will hang around long enough to be detected.
合成一種新元素非常復雜。GSI的粒子加速器中的鈦原子必須有足夠快的移動速度來克服自身質子間的斥力與锫質子間的斥力,卻又必須足夠慢,以防將還沒時間穩定下來的新合成元素119號撞裂。盡管需要良好的混合,Dullmann博士相信,在接下來的幾個月中,會合成一兩個119號元素的原子,并能穩定足夠長的時間以被檢測到。
That will be a feather in GSI's cap in its friendly competition with the Lawrence Berkeley National Laboratory, in California (after which berkelium is named) and the Joint Institute for Nuclear Research in Dubna, Russia (after which dubnium, number 105, is named). Number 110 is named darmstadtium, and these three laboratories are, between them, responsible for the creation of all the transuranics found so far-most notably plutonium, which was used to blow up Nagasaki in 1945 and thus end the second world war.
在與加利福尼亞州的勞倫斯伯克利國家實驗室(锫命名的由來之處)和俄羅斯杜布納的聯合核研究所(105號元素釷命名的由來之處)競爭的過程中,合成119號元素將會是GSI值得自豪的卓越成就。GSI實驗室合成了110號元素,它也因此被命名為鐽;不止如此,這三所實驗室合成了迄今為止所有的超鈾元素。尤其引人注目的是钚,正是1945年在長崎爆炸的原子彈所使用的元素,也因此結束了第二次世界大戰。
The islands of the blessed.
有福之島。
Modern transuranic research is more peaceful than it was in the 1940s. Indeed, sceptics might wonder at the value of creating new elements a mere atom or two at a time for little reason other than to show that it can be done. There is, however, method in the madness. Just as technetium and promethium are islands of instability in parts of the periodic table which are not normally radioactive, so many physicists believe that in the unstable part at the bottom there lies an island of stability. Their prediction is that nuclei containing 184 neutrons (which would have atomic numbers in the 120s) will hang around for sensible amounts of time-possibly as long as several million years. That would at least give them a chance to be useful.
現代超鈾元素的研究比上世紀40年代和平了許多。的確,懷疑論者會質疑,創造一個新的元素一次僅一、兩個原子,只為展示它可以被合成,這樣做究竟意義何在。然而,這樣的舉動雖看起來怪異,實際上卻也合乎情理。正如周期表中的锝、钷是在通常沒有放射性的地方出現的不穩定島,所以許多物理學家認為,在不穩定部分中實際潛藏著穩定元素島。他們預測,有184個中子的原子核(原子序數應在120-130之間)會穩定出現足夠長的時間——也許是幾百萬年。這起碼使它們可以被利用變為可能。
Dr Dullmann's version of element 119 will not quite be there. It will have only 177 neutrons. But if it can be made, it will be a stepping stone towards the fabled island of stability, which is generally agreed to be a worthwhile destination. That it will be one in the eye for the Americans and the Russians as well is purely coincidental.
Dullmann博士所述的119號元素也并不能達到穩定島,它只有177個中子。但如果119號元素可以合成,將會是走向傳說中的穩定島的一個臺階,而穩定元素島是公認的有價值的目的地。這也將會同時給美國人和俄羅斯人帶來打擊。











